Download PDF
Introduction
Pyrogens, a heterogeneous group of fever inducing contaminants, are comprised of microbial and non-microbial compounds; e.g. endotoxin (lipopolysaccharide, LPS) produced by Gram-negative bacteria, lipoteichoic acid (LTA) from Gram-positive bacteria, bacterial DNA (CpG-motive), endogenous pyrogens, virus and fungi particles or even fragments of packaging materials, plastic and dust. Contamination with pyrogens in pharmaceutical products, biotherapeutics and on medical devices can lead to life threatening fever reactions. To ensure consumer safety, pyrogen testing is mandatory for parenteral products and medical devices.
Until recently, Wickham Laboratories Ltd have offered two compendial methods of pyrogen testing, the Rabbit Pyrogen Test, RPT, (EP 2.6.8, USP 151) [1, 2] and the Bacterial Endotoxin Test (Limulus Amebocyte Lysate, LAL, EP 2.6.14, USP 85) [3, 4]. Both methods feature limitations in the scope of their application. RPT is not suitable for products such as radiotherapeutics, chemotherapeutics, analgesics, steroids, cytokines, dopamine and immunosuppressive agents while LAL cannot be applied to drugs that interfere with the clotting system, cell therapy products, endotoxin-binding plasma components or products containing glucans to name a few. Furthermore, LAL, unlike RPT, detects only endotoxins from Gram-negative bacteria as the mechanism of action does not reflect the inflammatory response of a mammalian organism but instead is based on the clotting reaction of the haemolymph of the horseshoe crab. Inability to detect non-endotoxin pyrogen contaminants precludes the BET/LAL method from being a complete in vitro alternative to the RPT.
Challenge
Wanting to meet the expectations of clients who required pyrogen testing for samples that could display compatibility issues with both RPT and LAL method, and following our commitment to expand our portfolio of in vitro alternatives to in vivo methods, we recognised the need for another pyrogen detection method. A non-animal in-vitro alternative that would be capable of detecting both endotoxin and non-endotoxin contamination appeared to be a perfect solution.
The Monocyte Activation Test (MAT) (EP 2.6.30, FDA Q&A June 2012) [5, 6] was developed on the principle of the human immune system response: when challenged by the pyrogens entering the body or coming into contact with the blood stream, the host’s innate immune defence mechanisms cause the monocytes / macrophages to produce prostaglandins and pro-inflammatory cytokines such as interleukin-1 (IL-1), interleukin-6 (IL-6) and tumor necrosis factor-α (TNF- α). The initiated pro-inflammatory signalling pathway can cause fever resulting in severe inflammation, organ-failure and even death. Mimicking this biological response, Merck’s PyroDetect System (Merck KGaA, Darmstadt, Germany) applies the principle of MAT by utilising cryo-preserved human whole blood as a source of monocytes and determines pyrogenicity of the tested sample by measuring the Interleukin-1β production in an immunological assay (ELISA) (Fig. 1). PyroDetect System detects both Gram-positive (non-endotoxin) and Gram-negative (endotoxin) contamination alongside other biological (virus, fungi) and non-biological pyrogens.
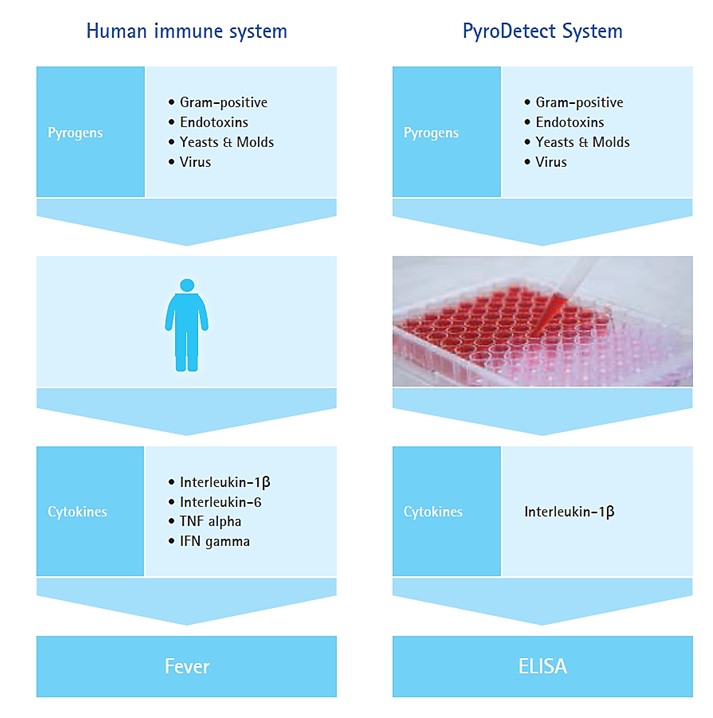
Figure 1. Principle of PyroDetect System (MAT). Graphic from the PyroDetect brochure “The human(e) alternative in pyrogen testing” courtesy of Merck KGaA, Darmstadt, Germany.
MAT has been an official test of the European Pharmacopoeia since 2010. As a compendial method, it requires verification in order to determine whether, under the actual conditions of use and for the specified substance or drug product matrix, the procedure could be used for its intended purpose. The monograph 2.6.30. specifies the requirement for preparatory tests that have to be incorporated into the verification protocol in order to ensure both precision and validity of the test.
Validation
In brief, as part of the verification protocol, we evaluated a number of performance characteristics selected due to their representation of the method’s critical aspects, e.g. accuracy, precision, specificity, detection limit and quantitation limit, range and robustness. Moreover, following chapter 2.6.30 of European Pharmacopeia, the preparatory tests involving the confirmation of the criteria of the standard curve and test for interfering factors were conducted. Due to the underlying mechanisms of the human fever reaction stimulated by pyrogens (both endotoxin and non-endotoxin based) [7, 8] and overwhelming scientific evidence of the MAT method detecting a broad range of pyrogens, Gram-negative and Gram-positive, alongside other biological and non-biological pyrogens [for review see 9], the evaluation of the detection of non-endotoxin contamination by MAT was omitted. This decision was further substantiated by the demonstration of the detection of a broad spectrum of pyrogens using the PyroDetect System itself [10].
Following initial successful verification of the PyroDetect kit for the purpose of pyrogen detection, each new product intended for testing will be subjected to preparatory testing (product specific validation). Firstly, the appropriate method (A – quantitative, B – semi-quantitative or C – reference lot comparison test) will be established based on the product’s specific characteristics. Secondly, the test for interfering factors will be performed on at least three separate production batches in order to confirm that the test solution does not interfere with the test. Each test will also have to fulfil the criteria for the standard curve in order to be valid. Once the method is confirmed to be suitable for the pyrogen detection in the given test solution, the SOPs for the routine execution of the method will be developed and approved. The documents will include the criteria for quality control checks and system suitability tests incorporated in each assay, such as criteria for the standard curve and interfering factors test. The analytical procedure will then be performed routinely for the given product. Any changes outside the scope of validation, e.g. change in the product’s formulation, will result in new product specific validation.
Conclusion
Wickham Laboratories Ltd’s clients are now offered a Monocyte Activation Test as an in vitro alternative to the Rabbit Pyrogen Test. Our experienced scientists will establish the suitability of the method for each product, work on the development of an appropriate method and conduct a GMP product specific validation according to European Pharmacopoeia. We hope to continue to meet the expectations of our clients and improve our service portfolio with relevant in vitro alternatives to in vivo tests while offering highest quality scientific and analytical service.
References
1. European Pharmacopoeia 8.8 (2016), 2.6.8. Pyrogens.
2. United States Pharmacopoeia 39 – NF 34 (2016), <151> Pyrogen Test.
3. European Pharmacopoeia 8.8 (2016), 2.6.14. Bacterial Endotoxins.
4. United States Pharmacopoeia 39 – NF 34 (2016), <85> Bacterial Endotoxins Test.
5. European Pharmacopoeia 8.8 (2016), 2.6.30 Monocyte-activation test.
6. FDA (2012) Guidance for Industry. Pyrogen and Endotoxins Testing: Questions and Answers.
7. Rockel C., Hartung T. (2012), Systematic review of membrane components of gram-positive bacteria responsible as pyrogens for inducing human monocyte/macrophage cytokine release. Frontiers in Pharmacology 3:56.
8. Henderson B., Wilson M. (1996), Cytokine induction by bacteria: beyond lipopolysaccharide. Cytokine 8(4):269-300
9. Hasiwa N, Daneshian M, Bruegger P, Fennrich S, Hochadel A, Hoffmann S, Rivera-Mariani FE, Rockel C, Schindler S, Spreitzer I, Stoppelkamp S, Vysyaraju K, Hartung T. (2013), Evidence for the detection of non-endotoxin pyrogens by the whole blood monocyte activation test. ALTEX 30(2):169-208.
10. Merck KGaA, Darmstadt, Germany (2014), Detection of a broad spectrum of pyrogens with the PyroDetect System, Application Note.


