Rapid and Traditional Methods in the Contract Microbiology Laboratory
In the world of contract testing, we are bound by regulatory guidelines and high client expectations. We perform the necessary safety and performance testing of pharmaceutical and medical device products that will be used by the general public. It is therefore vital that we work to prescribed guidelines with our performance under constant review through audits by regulatory bodies and our clients. Most people associate science with cutting edge techniques and technologies in the laboratory setting. Unfortunately, though, most microbiology laboratories involved in contract testing perform very traditional and prescribed methods set out by regulators and requested by clients, leaving very little room for innovative technologies and techniques.
Uptake of new techniques and technologies into standards such as ISO, Ph. Eur, USP, JP etc. is often slow, which can be frustrating for scientists and clients alike. Advisory boards and other interested parties can take many years to reach a consensus on how testing should be performed in these standards to meet the discerning eye of the regulatory inspector. However, there have been recent efforts to bring on new technologies into some of the standards, as well as efforts by advisory boards and companies to validate new rapid microbiology methods.
RMMs are an expanding area, with limited uptake by scientists governed by GMP guidelines but far better adoption in other sectors, for example, “hygiene” type monitoring in the food industry, as well as in the clinical microbiology setting, where time is the most valuable commodity. As an expanding area of microbiology, RMMs are vast and diverse in nature and are now being looked at seriously by microbiologists as viable alternatives to the traditional methods we are accustomed to.
There are pros and cons to any method used in testing, and RMMs are no exception to this. Some of these pros and cons have been set out for RMMs versus traditional microbiology methods in Table 1.
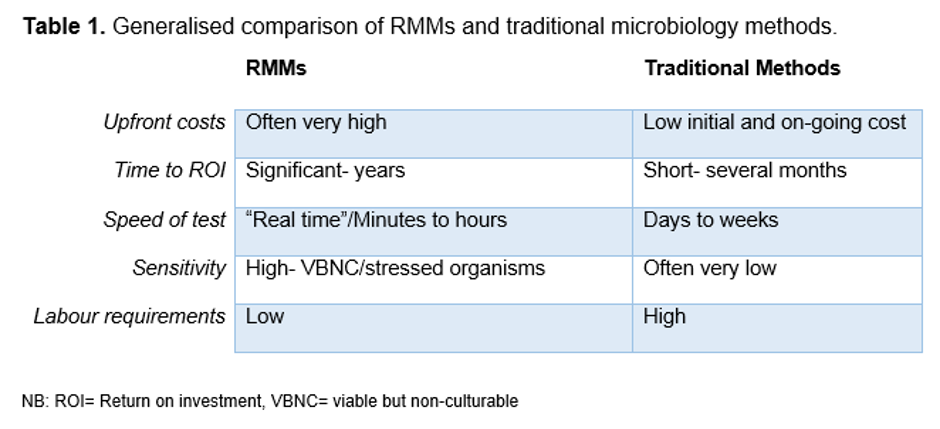
RMMs can bring significant cost and time savings (Table 1), though the most common barriers to entry with RMMs are the initial cost of the investment and the costs and/or perceived difficulty in bringing these tests in-house in a compliant manner1. The costs of RMMs may be several thousand pounds to hundreds of thousands of pounds to purchase and implement, depending on the complexity of the equipment in question. As a result many companies will simply continue with existing traditional microbiology methods and/or contract the work out to a contract testing laboratory or specialist lab.
Most companies investigating the use of RMMs in their processes are looking to significantly reduce their costs through increased throughput, time to result and reductions in labour. There is an increasing demand to get product to market more quickly, and more importantly to get them to market safely. In order to use RMMs to do so, microbiologists have to be convinced of their ability to detect and identify microorganisms, even more so than in traditional methods. This means potentially screening most, if not all, products in a batch prior to release, or even quicker turnarounds on products that rely on parametric release. In addition, many pharmaceutical and medical device manufacturers are also unsure how they cope with the VBNCs and stressed environmental organisms that can now be picked up by more sensitive methods, and are reluctant to address the changes that need to be made in-house to accommodate this shift1.
The Sterility Test
The most obvious microbiological test that would benefit favourably by using a RMM is the sterility test. The traditional sterility test has a 14-day incubation period which prevents timely release of product to market, or subsequently means a product may have already been in use before a test result is confirmed, e.g. in short shelf-life products. Only a small number of samples within a batch of product are tested, meaning the number of samples tested is often not statistically significant (varies depending on product and batch size) and may mean a contaminant is present but simply not detected2. The product is, like in many microbiological tests, tested to destruction, meaning it cannot be investigated if a suspect result is found. In the sterility test, the end result is measured by turbidity determination with the naked eye, which is open to subjectivity and any turbidity caused by product presentation could present as a false positive result. The sterility test method also uses two liquid based broths at set temperatures (20-25°C and 30-35°C), which “theoretically” covers the growth requirements of a large number of organisms. Yet we know that VBNC organisms, slow-growing organisms and organisms with complex culturing conditions will simply not be detected using this method2.
Current RMMs
Traditional microbiology still rules in today’s laboratories. Microbiologists still conduct many standard assessments using Gram or other differential stains, colony morphology, cell morphology, selective media, manual counts and biochemical assays such as API and Vitek 2 to determine the presence (or absence), number and identity of microorganisms that they culture.
There are many types of RMMs, each with different parameters and result formats. There are three main categories; qualitative, quantitative and identification.
Qualitative RMMs detect the presence of microorganisms in products. There are a number of tests currently available that measure changes in impedance, CO2 (via colour change in media) or pressure (headspace pressure) that signal microorganism growth. There are also methods such as polymerase chain reaction (PCR), flow cytometry and endotoxin tests (Limulus ameobocyte assay test) that can be used to more rapidly detect presence of microorganisms (or related bacterial endotoxins in the case of LAL). ATP measurements are also becoming more commonplace and have been used very successfully in hygiene monitoring to detect contamination. There is some difficulty perceived when comparing RFUs (relative fluorescence units) yielded by the ATP assays with CFUs (colony forming units) detected by traditional means, and how exactly these relate to one another1.
Quantitative RMMs are capable of providing a numerical value on the microorganisms present in a known sample unit. This is useful across the spectrum of microbiological tests. PCR is again useful as it is capable of quantifying as well as detecting the presence of microorganisms (commonly RT-PCR) in a matter of hours. There are also other methods that utilise direct detection of microorganisms using digital imaging of microcolonies (far too small to be seen with the naked eye) growing on solid media or filters as in direct laser scanning, light scattering, ATP bioluminescence and auto fluorescence methods. Methods such as flow cytometry and raman spectroscopy are also coming to the fore in quantitative RMMs, providing a much quicker time to result than the standard incubation and detection procedures used in most microbiology laboratories1,3.
Traditional biochemical tests can still be found in microbiological identification and have been refined over the years to be semi-automatic and less reliant on the user’s interpretation of results. As a result, these tests are able to produce results in as little as a few hours but can often take as long as a couple of days depending on the microorganism’s growth requirements and “stress” status.
An alternative to the more traditional microbiological tests is matrix-assisted laser desorption ionisation time of flight mass spectrometry (MALDI-ToF). This is a different phenotypic identification method used to determine the identity of a microorganism on the basis of a protein profile or ‘fingerprint’. It is relatively inexpensive to run samples in the MALDIToF equipment and results are available in minutes, but there is a large outlay cost that is seen to be prohibitive for small throughput in smaller laboratories.
PCR or nucleic acid-based methods can be used to identify organisms based on the 16s rRNA gene (or 16sDNA) in bacteria or the 26s rRNA gene, ITS or D2 region in fungi. They can provide a result in a matter of hours, most likely an overnight run, but can identify to a very distinct level (type or sub-type) so are used commonly in determining the exact source of sterility failures or serious contaminant route cause analysis3.
Guidance on Validation of RMMs
As discussed previously, a small number of companies have been successful in validating alternative methods to the acceptance of the regulatory authorities. The main goal in RMM validation is providing proof that the RMM exceeds or is at least equivalent in a number of areas as per 21 CFR <610.9> and other regulatory authority guidelines4.
There are now even standard methods in the compendial procedures, for example in the microbiological control of cellular products Ph. Eur 2.6.275), which use RMMs. This would seem to derail the point that most companies make regarding regulatory bodies not being fond of RMMs. On the contrary, regulatory authorities want more robust, sensitive and timely tests to prevent the devastating and often high-profile cases of contamination of pharmaceuticals and biologicals that have been seen in recent years. Many will remember the multi-state incidents in the United States in 2012 of deadly meningitis caused by mould contamination in steroid injections.
There are a number of documents that provide outline guidance on how to go about validating a RMM, such as the PDA Technical Report 336, Ph. Eur 5.1.6, USP Chapter <1223> and FDA CBER Draft guidance document, to name but a few. The validation methods are not wholly dissimilar to those for validating other methods in the GMP environment, and all of the guidelines are very similar to each other in their requirements for validation of RMMs. The regulatory guidelines may require a combination or all of the following to be completed, depending on the nature of the RMM in question and its purpose; testing for accuracy, precision, specificity, limit of detection, limit of quantification, linearity, range, ruggedness, robustness and equivalence testing6.
Conclusion
RMMs are definitely the future of microbiology and we are seeing rapid advancement in the technology capable of detecting, quantifying and identifying microorganisms. It is bringing with it some uncomfortable truths about how much we know about contaminants, VBNC and the downfalls of some of the traditional methods. Companies will have to look at this new data and interpret what it means to them and their product or process. Many laboratories will also have to branch out into techniques and new methods different to those they have become accustomed to over the years, and it is well known that old habits are notoriously hard to break. Many also believe there is value in keeping the traditional methods in microbiology alive, such as the Gram stain, identification by appearance, smell and biochemical reactions, and fear this will be forever lost when RMMs are used more widely. This is unlikely to be the case as many methods require some understanding of microorganisms, even at a basic level of bacteria, yeast, fungi, Gram positive, Gram negative, etc. to perform the tests and to understand what remedial steps have to be taken upon identification or detection of problematic microorganisms in a process or product. We will continue to rely on more traditional methods to determine such things as what decontamination processes should be used, whether processes should be changed, and whether personal protective equipment (PPE) should be worn to prevent cross-contamination during a process.
Acceptance of RMMs is slow in the pharmaceutical environment, but will be driven by the regulators championing these technologies and working with companies to help them overcome some of the hurdles along the way. Companies working on lean initiatives will also have to look hard at their processes and the bigger picture of the potential benefits in cost savings, labour reduction and most importantly increased patient safety in order to bring RMMs to reality in a way that works for their process and product.
Hopefully some of the early adopters of RMMs with encouragement of the regulatory bodies will facilitate uptake and confidence in RMMs. However, given the relatively slow change in many of the traditional microbiology methods over the past 70 or more years, it seems the likes of the traditional sterility test will be with us in current form for some time yet.
References
1. PHSS. Bio-contamination Technical monograph No. 20 Bio-contamination characterisation, control, monitoring and deviation management in controlled/GMP classified areas. (2014)
2. Jeanne Moldenhauer. Rapid Sterility Testing. (2011)
3. http://rapidmicromethods.com/files/matrix.php, visited 6 August 2015.
4. 21 CFR <610.9> Equivalent methods and processes (2014)
5. European Pharmacopoeia Ph Eur 2.6.27 Microbiological control of cellular products.
6. PDA. Technical Report No. 33. Evaluation, validation and implementation of new microbiological testing methods. (2013).
Editorial
Editorial on Rapid vs Traditional Microbiological Methods Published in IPI
Posted 7th October 2015 by Wickham Micro
We are pleased to distribute the following editorial on rapid versus traditional methods which was recently published in IPI:


